RGN-259
 |
RegeneRx’s highest priority is the development of RGN-259, a Tβ4-based sterile and preservative-free eye drop, as a novel treatment for dry eye disease and neurotrophic keratitis (NK), a persistent corneal defect caused by diabetes and the herpes zoster virus, among other pathologies. NK is an orphan disease as there are fewer than 200,000 cases in the U.S. We previously received orphan drug status from the U.S. FDA for RGN-259 for the treatment of NK. Our U.S. joint venture, ReGenTree LLC, recently initiated phase 3 clinical trials in the U.S. and EU designed to confirm previous phase 3 results.
Our second priority is the treatment of dry eye disease (DED). The conclusions from our analyses in over 1,600 clinical patients were that while RGN-259 did not meet the pre-specified coprimary endpoints of the trials with statistical significance, RGN-259 did demonstrated statistically significant improvements in both signs and symptoms of dry eye disease in various patient populations and after one and two weeks of treatment when measured across all three phase 3 clinical trials. The product candidate’s excellent safety profile was also confirmed. Currently, the goal is to await the results of the phase 3 NK trials, prior to initiating further phase 3 clinical trials in patients with DED.
NEUROTROPHIC KERATITIS
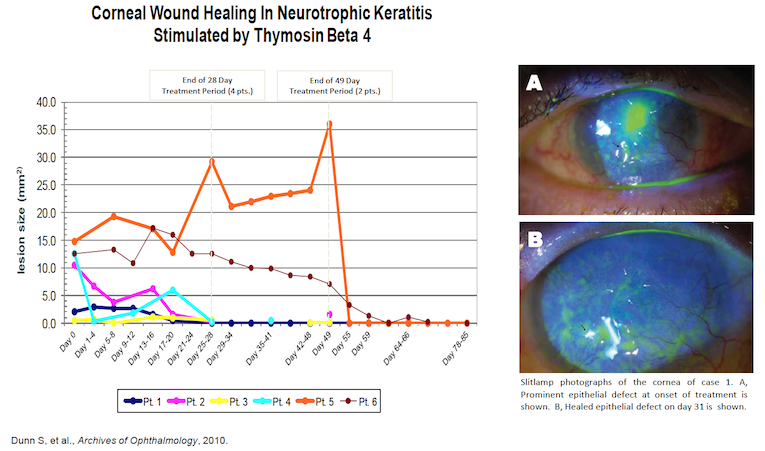
Initially, RGN-259 was used to treat NK patients with non-healing ulcers under a “Compassionate Use” IND. The physician treated 4 patients for 28 days and 2 additional patients for 49 days with RGN-259. The eye ulcers either completely healed or demonstrated significant improvement by the end of treatment or shortly thereafter (See figure below). During the 30-day follow-up period patients remained healed even though the treatment regimen had been completed.
Subsequently, clinical results in patients with NK treated with RGN-259 demonstrated efficacy in a small number of patients (18) in the first double-masked, placebo-controlled phase 3 clinical trial (SEER-1). Top line results for the NK trial (SEER-1) were reported in May 2020. The trial recruited, treated and analyzed 18 patients. Six out of 10 patients in the RGN-259 treated group and 1 out of 8 patients in the placebo treated group achieved complete corneal healing in 4 weeks. In terms of the primary endpoint, "ratio of corneal wound healed patients after four weeks' administration," the statistical difference was slightly over 0.05 (p = 0.0656, Fisher's exact test), due to the limited number of patients in each group. This strong trend likely would have reached a statistically significant p value of <0.05 had more patients been entered into the trial. When another statistical analysis was used to analyze the same primary endpoint (Chi square test), there was statistical significance, p = 0.0400, even with the limited number of patients.
In addition, in a pre-specified secondary endpoint evaluating corneal epithelial healing at day 43 (two weeks post-treatment) and the durability of RGN-259 treatment, there was a clear statistical difference using the Fisher's exact test, p = 0.0359. Several other efficacy parameters were either highly significant or strongly trending toward statistical significance in the RGN-259 group indicating the depth of patient response to RGN-259. These results demonstrated the efficacy of RGN-259 in NK, despite the small number of patients. As expected, it was well-tolerated and there were no safety issues.
Two phase 3 clinical trials are now underway in the United States and Europe based on the results of SEER-1, (SEER-2 and SEER-3). ReGenTree aims to significantly shorten the clinical development period by conducting the two phase 3 clinical trials simultaneously, rather than sequentially. The double-masked, placebo-controlled trials will be conducted by administering RGN-259 or placebo eye drops for four weeks to approximately 70 NK patients in each trial, with a primary endpoint of "complete corneal healing." Considering it is a rare disease, to accelerate patient recruitment ReGenTree has recruited over 30 clinical centers to participate in the studies, with 13 currently active in the U.S. Initial results are targeted for early 2024.
Neurotrophic keratitis offers a relatively large market opportunity as a rare disease with about 20,000 patients a year in the United States. Oxervate, from Dompé Farmaceutici in Italy, is currently the only approved treatment for NK in the United States and is one of the most expensive pharmacy drugs in the U.S. with a retail price of $48,498 per month for a two-month treatment course, according to industry newsletters.
DRY EYE Disease
The ARISE clinical trials are a series of three phase 3 clinical trials in over 1,600 patients, conducted in multiple medical centers across the U.S., assessing RGN-259 for the treatment of dry eye disease. As part of the process to fully understand patient data and the effects of RGN-259 compared to placebo we evaluated various subgroups of DED patients within ARISE-3 and pooled the data from all three ARISE clinical trials. Pooled data combines the patient data from all ARISE trials to analyze the results, which may also be compared to the results of each individual trial.
The key results were:
(1) While the pre-specified co-primary endpoints were not met, ARISE-3 provided statistically significant improvements with RGN-259 in a specific and common dry eye symptom, ocular grittiness, compared to placebo:
a. observed one week after treatment;
b. observed two weeks after treatment;
c. observed two weeks after treatment after dry eye patients were stressed in the CAE model; and
d. In several questions using the ocular surface disease index scale (OSDI), a different symptom assessment methodology.
(2) In ARISE-3, outside of the prespecified endpoints, statistically significant differences were seen in central corneal fluorescein staining, a sign of dry eye, at two weeks after treatment with RGN-259 in a subpopulation of patients;
(3) In a pooled sub-population of all three ARISE trials, statistically significant sign differences were also seen in central corneal staining;
(4) In ARISE-2 and in the same patient population within the pooled group of all three ARISE trials, statistically significant differences were observed in inferior corneal fluorescein staining, another sign of dry eye, at two weeks after treatment with RGN-259;
(5) RGN-259 acts rapidly;
(6) RGN-259 continued to demonstrate that it is safe and well-tolerated in the treatment of dry eye.
As stated above, the data demonstrated statistically significant improvements in both signs and symptoms of dry eye disease in various patient populations and after one and two weeks of treatment when measured across three phase 3 clinical trials in over 1,600 patients. The product candidate’s excellent safety profile was also confirmed. The Company’s joint venture intends to continue development of RGN-259 for DED as soon as it successfully completes the phase 3 NK clinical trials.